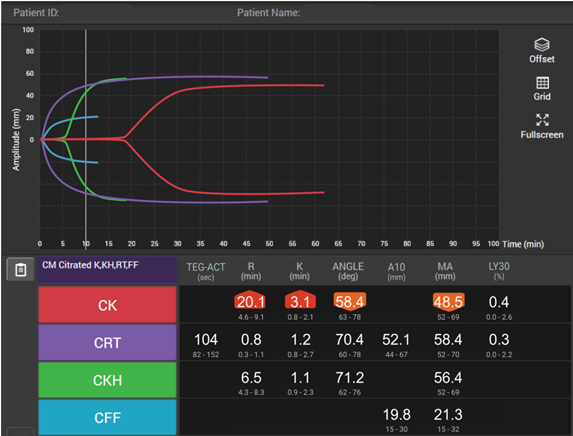
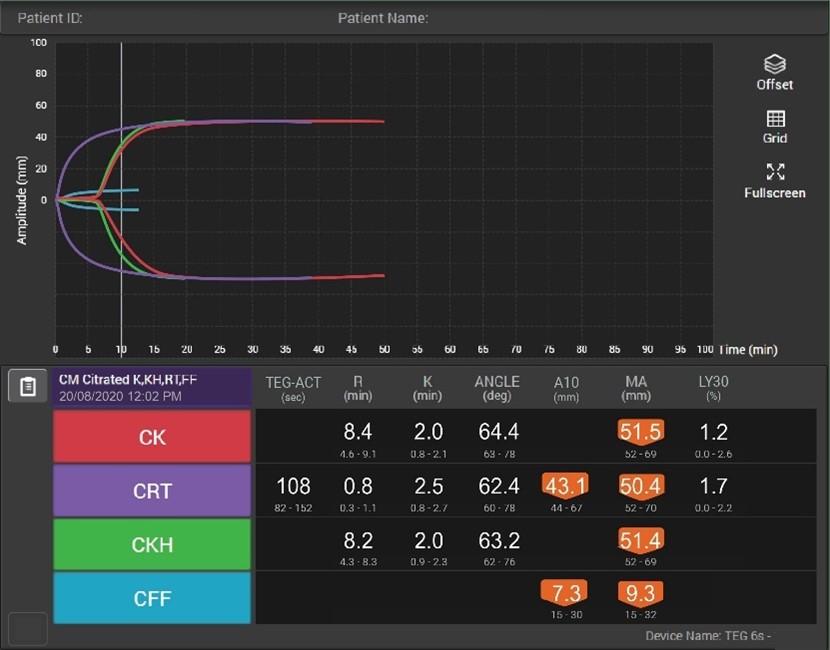
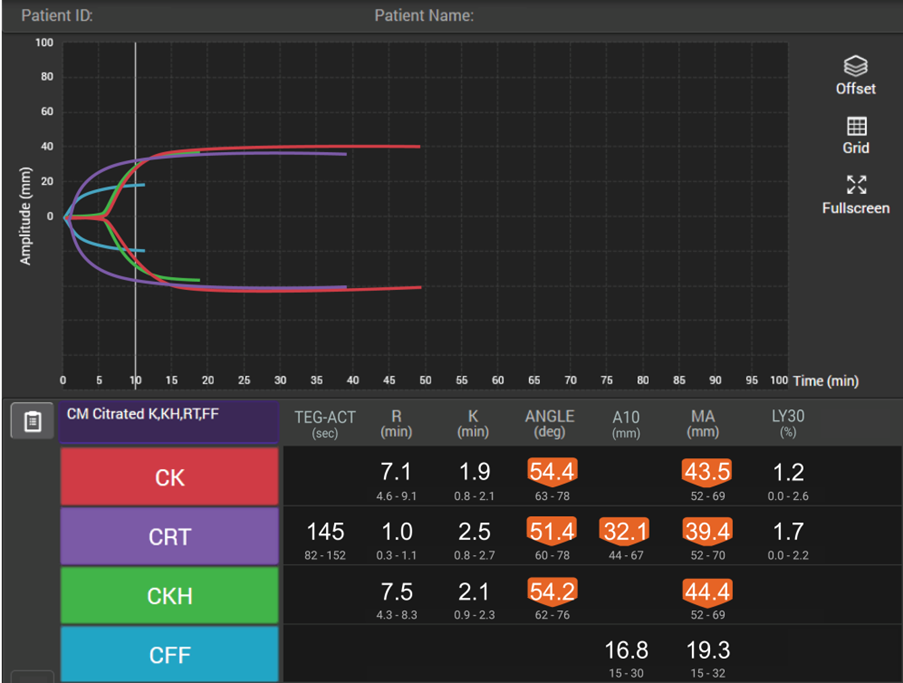
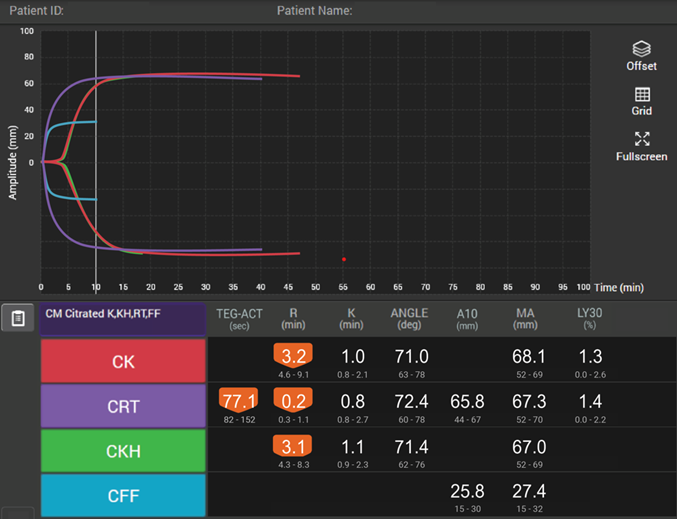
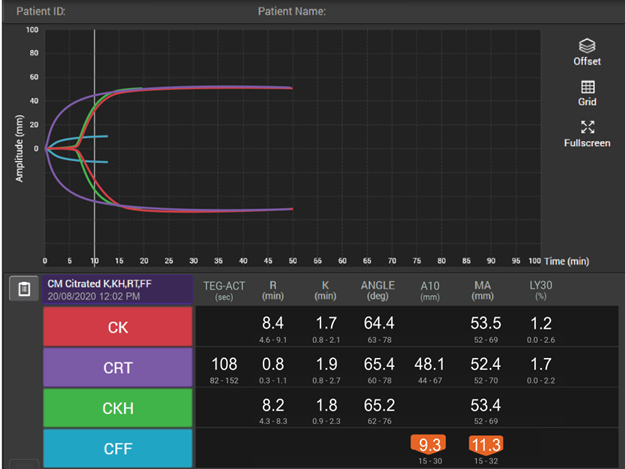
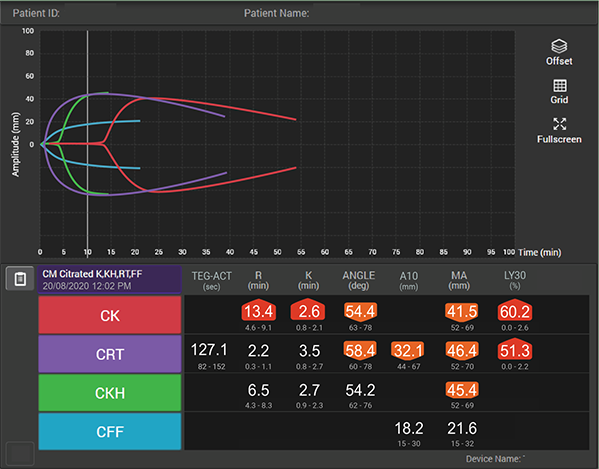
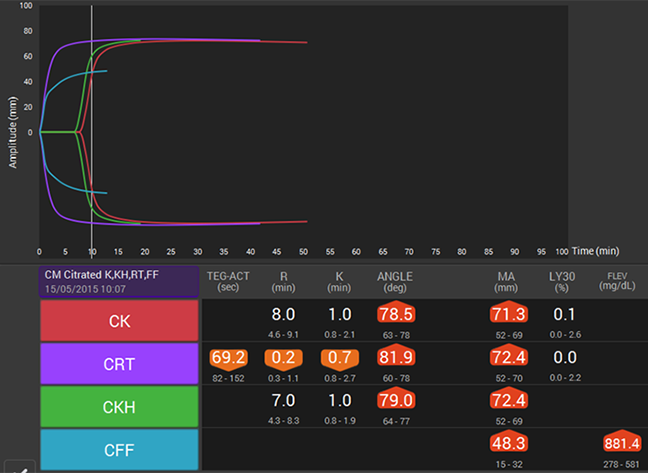
How viscoelastic testing can help
The use of viscoelastic testing can provide a rapid and accurate assessment of blood product requirements in the management of trauma-induced coagulopathy.4 Whilst conducting RCTs in the trauma setting is logistically difficult,39 the available data suggest that TEG-guided management in the trauma setting improves survival and can decrease the use of blood products (Table 5).
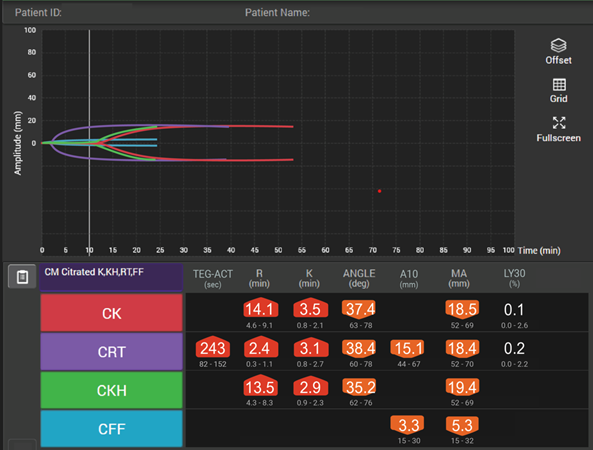
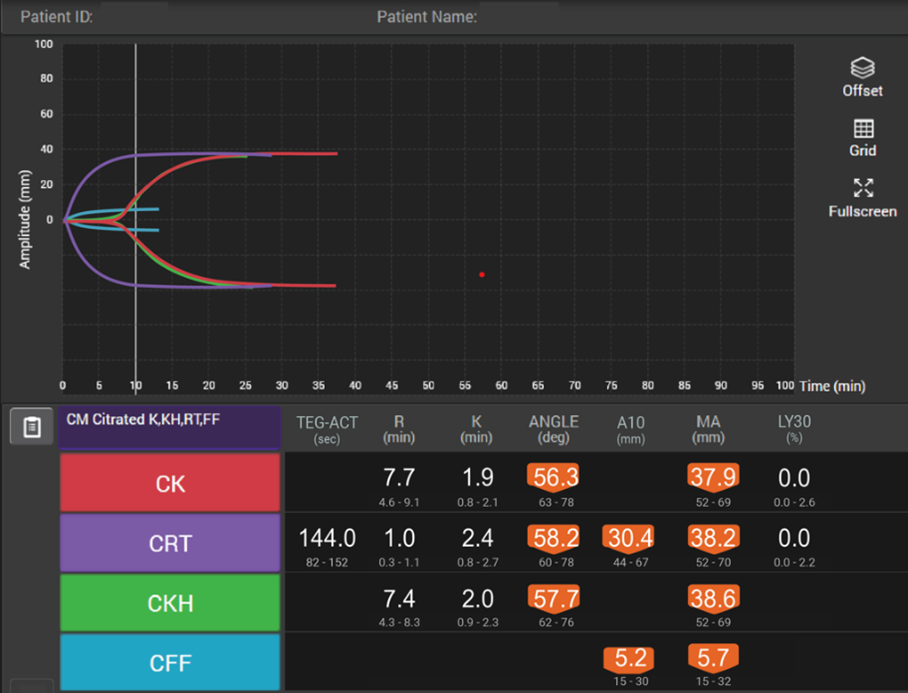
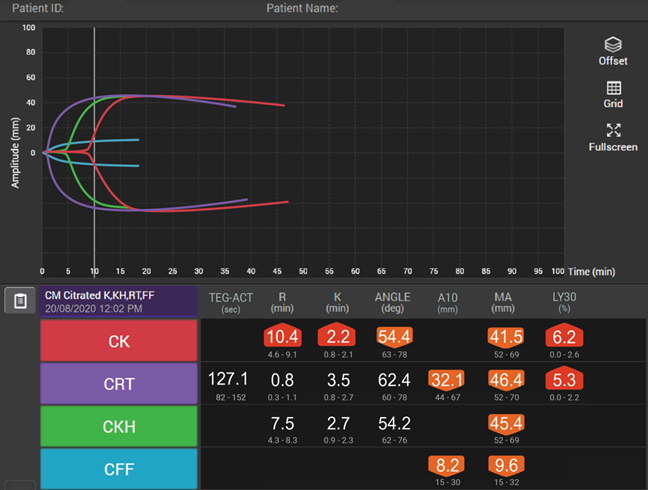
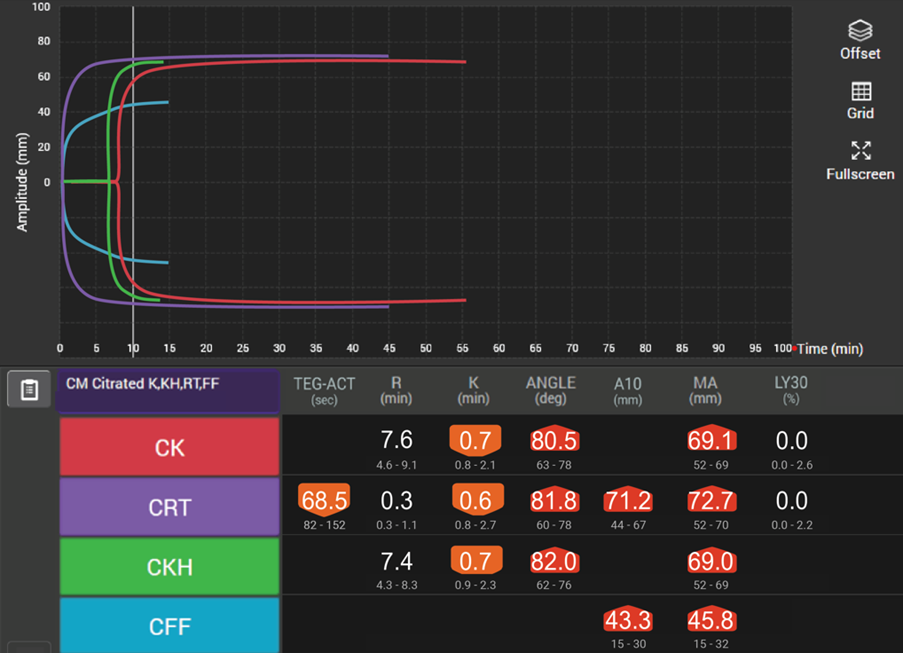
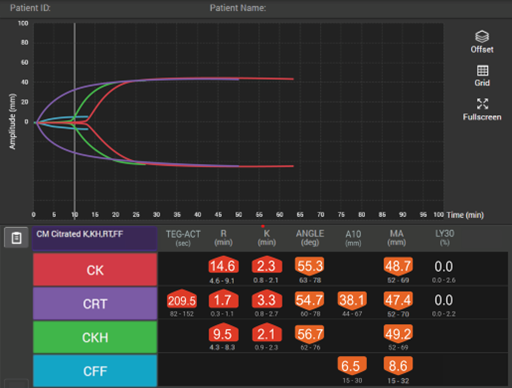
Viscoelastic testing can rapidly and dynamically evaluate the different components and stages of whole blood coagulation in trauma patients at the site-of-care and is thus highly reliable for use in this setting.35 TEG® analysis provides results more quickly than standard laboratory tests and provides more comprehensive results,40 making it more suitable for assessing coagulation status and blood product needs in an emergency.
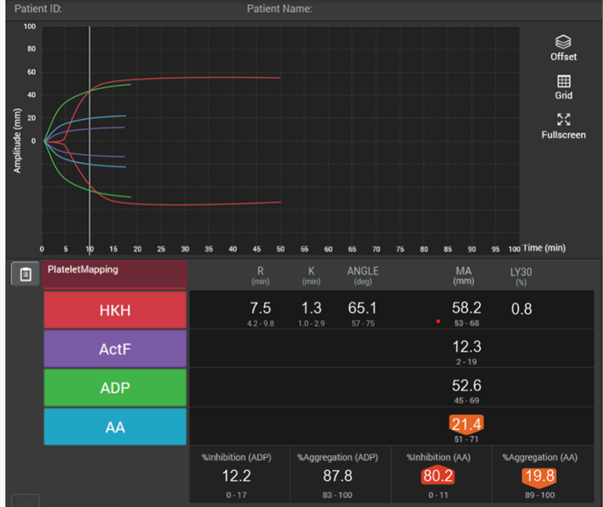
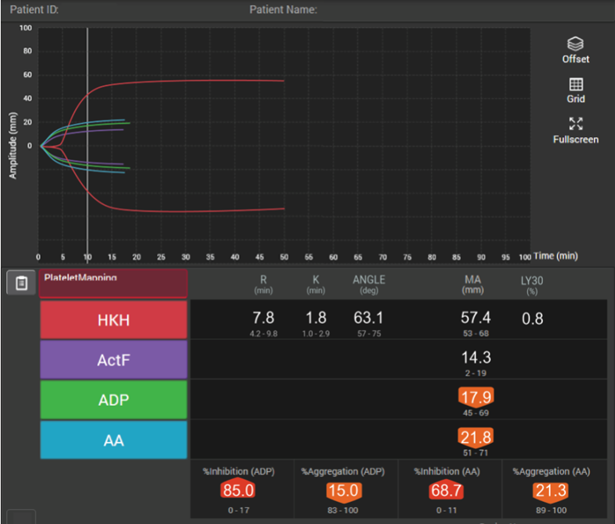
In patients with traumatic brain injuries, TEG® and TEG®PlateletMapping® can be used to characterise coagulopathy patterns,41 and TEG®PlateletMapping® assays may reduce platelet transfusions without a concurrent increase in clinically significant hematoma expansion in patients prescribed pre-injury antiplatelet therapy.5
Table 5: Viscoelastic testing versus standard coagulation testing in a trauma setting| | Study | Key Outcomes |
|---|
 | RCT (n=396)34 | There was no difference in proportion of subjects alive and free of massive transfusion 24 hours after injury when using major haemorrhage protocols based on viscoelastic haemostatic assays instead of conventional coagulation tests. |
 | RCT (n=111)4 | The use of a goal-directed, TEG®-guided massive transfusion protocol improved survival compared with a protocol guided by conventional coagulation tests. There were also reductions in plasma and platelet transfusions. |
 | Prospective, observational study (n=301)42 | Resuscitation algorithms based on TEG® were associated with reduced mortality (25% vs 11% at 30 days, p=0.002) and blood product wastage and were cost neutral compared with standard coagulation tests. |
The use of viscoelastic testing-guided algorithms in trauma is recommended by the European Multidisciplinary Task Force for Advanced Bleeding Care in Trauma in addition to or instead of standard coagulation tests.43 They also suggest the use of point-of-care platelet function devices as an adjunct to standard laboratory and/or point-of-care coagulation monitoring in patients with suspected platelet dysfunction. The British Society for Haematology advises that point-of-care viscoelastic testing has practical advantages for monitoring major haemorrhage, including speed of results and a set of parameters that assesses a global coagulation profile.36 However, they also state that, at present, the evidence base to guide practice is limited. The American College of Surgeons Trauma Quality Improvement Program massive transfusion in trauma guidelines recommend switching to a laboratory or point-of-care-based transfusion once major bleeding has been controlled and the rate of transfusion has slowed.44 In addition, the Eastern Association for the Surgery of Trauma provides a conditional recommendation for viscoelastic testing-guided transfusions over traditional coagulation parameters in adult trauma patients with ongoing haemorrhage and concern for coagulopathy;45 Finally, the French Working Group on Perioperative Haemostasis proposes that, in the setting of severe trauma, viscoelastic testing can be used for the early diagnosis of coagulopathy, and to indicate haemostatic treatment and to make clinical staff more aware of the severity of trauma.46